Ser ikke så verst ut, solid tailwind fra biotech i usa i går for Genmab
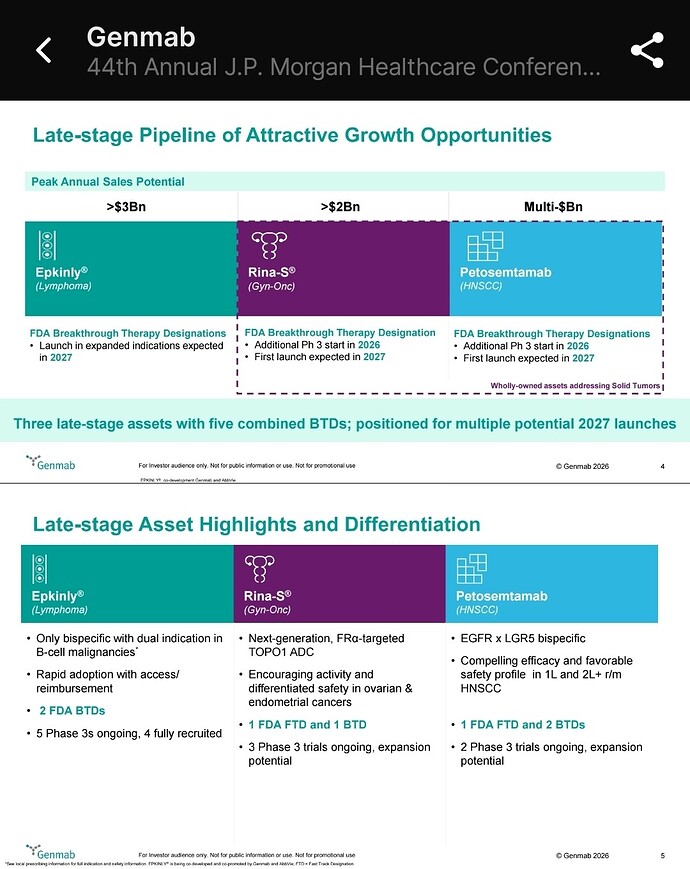
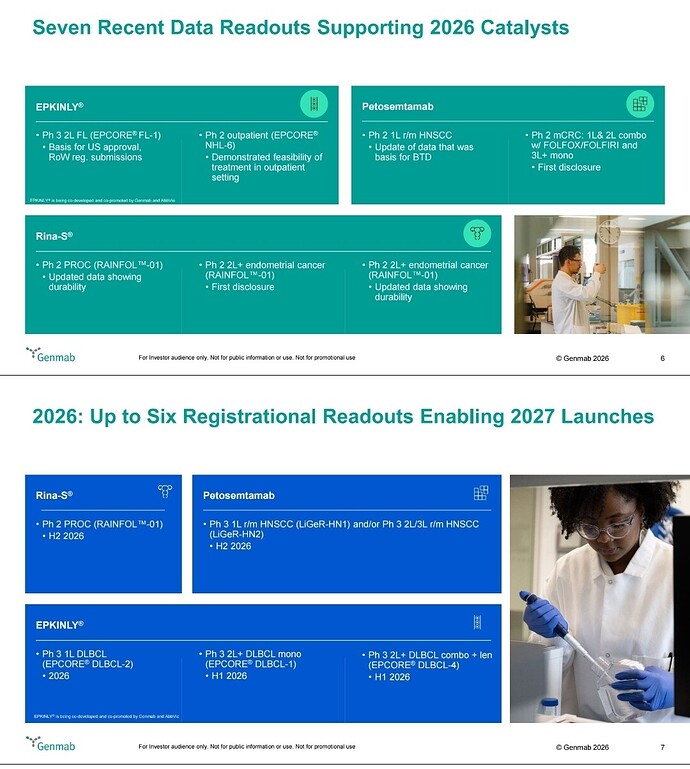
- EPKINLY plus rituximab and lenalidomide (EPKINLY + R 2 ) is the first and only bispecific-based therapy approved by the FDA for follicular lymphoma in the second-line setting
- In the Phase 3 EPCORE ® FL-1 trial, fixed duration EPKINLY + R 2 demonstrated significantly superior progression-free survival and overall response rates compared to standard of care R 2 , with approximately three out of four patients achieving a complete response
- This approval marks the third indication for EPKINLY and the first-ever FDA approval for a bispecific combination therapy in the lymphoma space
1 Like
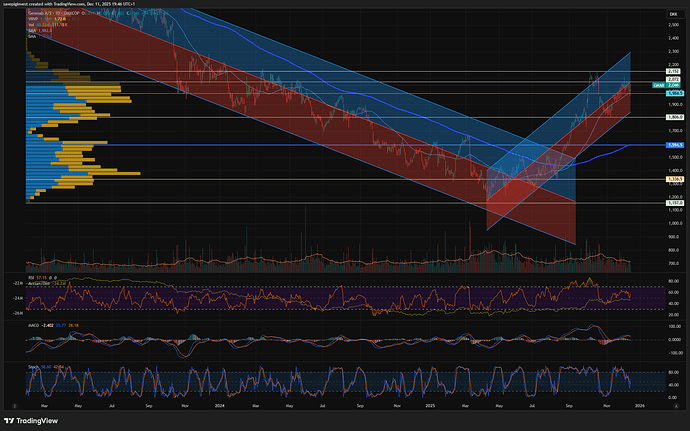
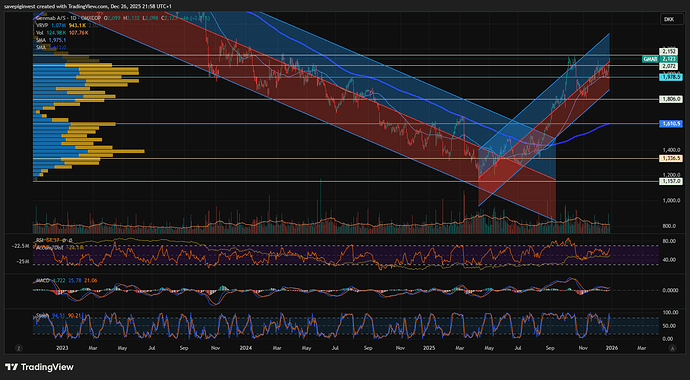
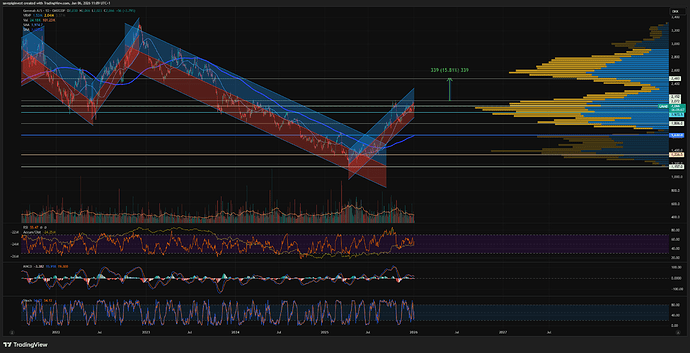
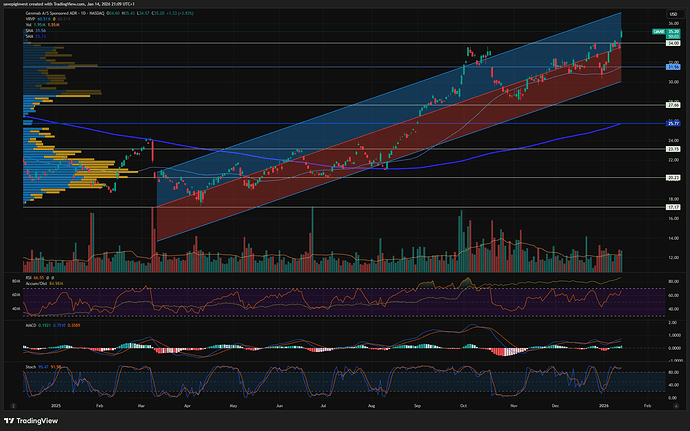
Stusset litt på hvorfor Genmab var så utrolig svak ift markedet, men ser nå at det ble nevnt på Shareville at de hadde en readout som kanskje skuffet litt.
Ikke stat sig OS på en Epkinly-readout her for litt siden. Forrige uke? Var ned 6-7% på det.
Meldingen kom på fredag, sikkert etter close for fredag var finfin.
Så raste den mye i går, jeg trodde det var en overreaksjon på trump, men skjønner nå det var en kombinasjon av skuffelse over meldingen og trump på en gang.
Når man får en sånn cocktail av sterk oppgang i det siste med en dobbel whammy på det fundamentale, så blir det store utslag
1 Like