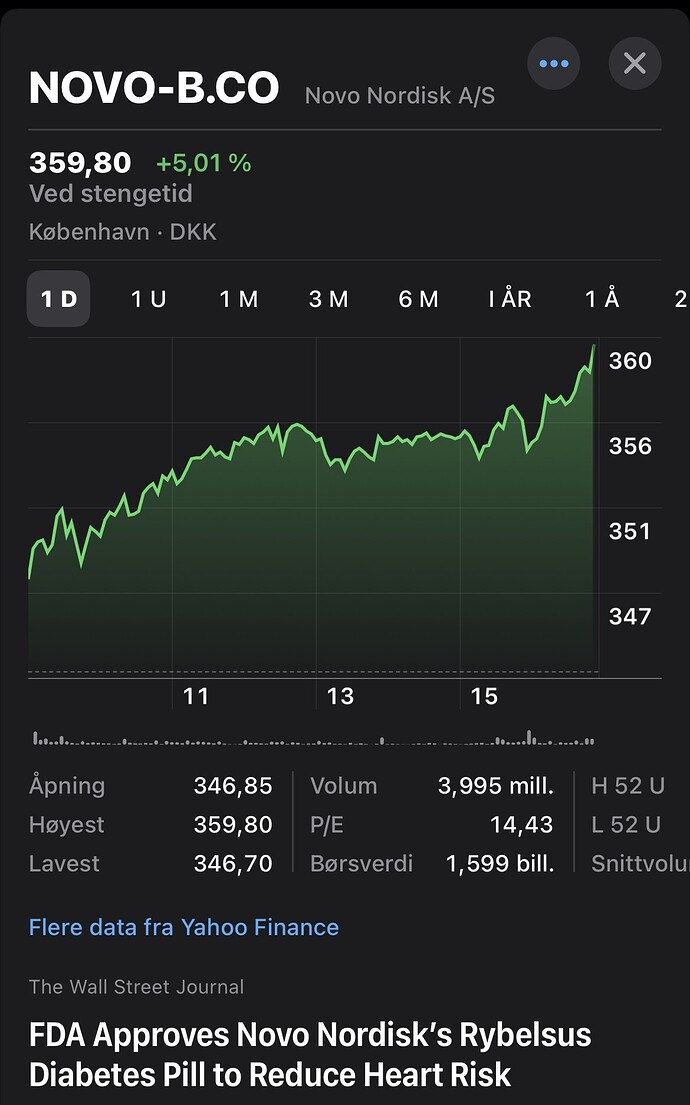
Novo Nordisk and Omeros announce asset purchase and license agreement for Omeros’ clinical-stage MASP-3 inhibitor zaltenibart (OMS906)
2025-10-15 14:30:41
- Zaltenibart has best-in-class potential across multiple rare blood and kidney disorders and will enhance Novo Nordisk’s Rare Disease portfolio
- Omeros is eligible to receive 340 million US dollars in upfront and near-term milestone payments, up to a total of 2.1 billion dollars including potential development and commercial milestones, plus tiered royalties on net sales
Bagsværd, Denmark and Seattle, US, 15 October 2025 – Novo Nordisk and Omeros Corporation (Nasdaq: OMER) today announced that they have entered into a definitive asset purchase and license agreement for the candidate drug zaltenibart (formerly OMS906) in clinical development for rare blood and kidney disorders.
Under the terms of the agreement, Novo Nordisk will be granted exclusive global rights to develop and commercialise zaltenibart in all indications. Omeros is eligible to receive 340 million US dollars in upfront and near-term milestone payments, up to a total of 2.1 billion dollars including potential development and commercial milestones, plus tiered royalties on net sales.
Zaltenibart is an antibody designed to inhibit MASP-3, a protein that acts as a key activator of the complement system’s alternative pathway. Dysregulation of the complement system, a crucial part of the immune system, has been shown to be involved in the pathophysiology of a number of rare diseases.
“Zaltenibart has a novel mode of action that could offer several advantages over other treatments for complement-mediated diseases,” said Martin Holst Lange, chief scientific officer and executive vice president of Research & Development at Novo Nordisk. “Novo Nordisk is in a strong position to build on the work done by Omeros to maximise the value of this asset and develop zaltenibart into a differentiated and potentially best-in-class treatment approach for a number of rare blood and kidney disorders.”
Omeros has reported positive phase 2 data for zaltenibart in paroxysmal nocturnal hemoglobinuria (PNH) - a rare, acquired blood disorder where the body’s immune system mistakenly attacks and destroys red blood cells, leading to low levels of healthy red blood cells and other complications. Zaltenibart has shown multiple potential advantages over other alternative pathway inhibitors in development or on the market, and it has been well tolerated and demonstrated an acceptable safety profile across all clinical trials to date.
“We are pleased to enter into this agreement with Novo Nordisk, a global leader in therapeutic innovation and development,” said Gregory A. Demopulos, M.D., Chairman and Chief Executive Officer of Omeros. “We look forward to Novo Nordisk levering its extensive expertise and global reach to unlock the potential of zaltenibart across alternative pathway indications. With Novo Nordisk driving the success of zaltenibart, Omeros remains focused on securing approval and commercialisation of narsoplimab this quarter and continuing to advance its robust development pipeline.”
Omeros retains certain rights to its preclinical MASP-3 programmes unrelated to zaltenibart, including the ability to develop and commercialise small-molecule MASP-3 inhibitors with limited indication restrictions.
Following closing of the transaction, Novo Nordisk aims to initiate a global phase 3 programme for zaltenibart in PNH and explore further development in a range of other rare blood and kidney disorders.
“With zaltenibart, we have a compelling opportunity to help a significant number of people living with rare blood and kidney disorders in the future and support our leadership ambition in this space,” said Ludovic Helfgott, executive vice president of Product and Portfolio Strategy at Novo Nordisk. “This agreement will build on Novo Nordisk’s heritage and enhance our existing Rare Disease portfolio with potential to drive additional growth in this business area.”
The transaction is subject to certain customary closing conditions, including applicable regulatory approvals, and is expected to close in the fourth quarter of 2025.
About zaltenibart
Zaltenibart (OMS906) is an investigational humanized monoclonal antibody that selectively targets mannan-binding lectin-associated serine protease-3 (MASP-3), the key and most upstream activator of the alternative pathway of the complement system and a critical component of innate immunity involved in host defense and immune regulation.MASP-3 is responsible for converting complement pro-factor D to its active form, factor D. With a low systemic circulation compared to other alternative pathway proteins and slow circulation clearance, MASP-3 is considered a highly attractive therapeutic target. Unlike inhibitors of C3 or C5, MASP-3 inhibition preserves the classical pathway function, which is critical to vaccine-induced immunity and defense against infections. Moreover, MASP-3 is not believed to be an acute-phase reactant, potentially offering another significant advantage for MASP-3 inhibitors like zaltenibart.
Zaltenibart has potential applications across a broad range of therapeutic areas and indications, including paroxysmal nocturnal hemoglobinuria (PNH), renal diseases such as immunoglobulin A nephropathy (IgAN), C3 glomerulopathy and atypical hemolytic uremic syndrome, and other immune and complement driven-disorders.
About Omeros Corporation
*Omeros is a clinical-stage biopharmaceutical company focused on discovering, developing, and commercializing first-in-class small-molecule and protein therapeutics for large-market and orphan indications, with a particular emphasis on complement-mediated diseases, cancers, and addictive or compulsive disorders. Omeros’ lead MASP-2 inhibitor narsoplimab targets the lectin pathway of complement and is under regulatory review by both the U.S. FDA and the European Medicines Agency for the treatment of hematopoietic stem cell transplant-associated thrombotic microangiopathy. OMS1029, a long-acting MASP-2 inhibitor, has successfully completed Phase 1 clinical trials. Zaltenibart (OMS906), Omeros’ MASP-3 inhibitor, is in clinical development for PNH and C3 glomerulopathy. Under a recently announced agreement, Novo Nordisk will acquire global rights to zaltenibart, including associated intellectual property and related assets. Omeros’ pipeline also includes OMS527, a phosphodiesterase 7 inhibitor in clinical development for cocaine use disorder, fully funded by the National Institute on Drug Abuse, as well as a growing portfolio of novel molecular and cellular therapeutic programs for oncology. For more information visit www.omeros.com
Bra dag for Omeros-investorer
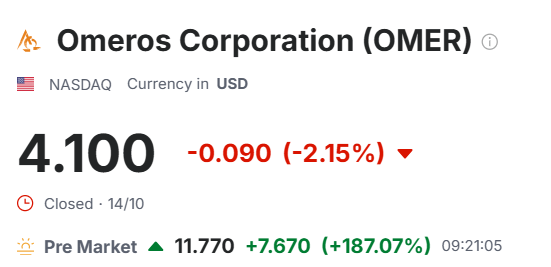
Ja men ruglete for de som har vært long der. Fra 25 dollar via 0.92(!) til dagens 10-11 dollar. Sånn er livet som biotek investor. Timing er alt.
Har han med hentesveisen slengt med leppa igjen nå?
Jepp…
Så da er det bare til å vente på TACO-effekten, tror jeg plukker tilbake litt aksjer her igjen.
Novo er under stort press. Finnes ikke verre enn usikkerhet og frykt for hva prisene blir og hva dette ender opp med. Trump er dyktig til å bevege markedene.
Godkjenning for oral semiglutide for fedme og topline data i alzheimer er ventet i Q4. To store katalysatorer hvis positivt.
De har vært veldig aggressive med oppkjøp og lisensdeals + masse oppsigelsene. På lang sikt positivt at selskapet investerer for fremtiden, men de bruker mye cash nå, og tar på seg mer gjeld. Mulig investorene ville heller foretrukket tilbakekjøp av aksjer. Ganske latterlig å tenke på at de brukte masse millarder på å kjøpe aksjer til det dobbelte av dagens kurs.
Trump er støy på mer støy.
Slankemedisin bør heller sponses av land en selskaper som skaper vidundermiddlene. Biotek svartmales, men er grunnen til verden blir eldre og eldre selvfølgelig må de få grovt betalt  bør oppfordres til videre forskning ikke trykke de ned.
bør oppfordres til videre forskning ikke trykke de ned.
Novo Nordisk to present new data in oral semaglutide 25 mg (Wegovy® in a pill*) and obesity pipeline at ObesityWeek® 2025
New data from OASIS 4 on oral semaglutide 25 mg to reveal insights on cardiometabolic health among adults with obesity or overweight
Additional trial insights into impact on ability to do everyday activities and women’s health among adults with obesity or overweight will also be shared
New CagriSema data from REDEFINE to be presented, highlighting innovative pipeline
Plainsboro, NJ and Bagsværd , Denmark, 20 October 2025 – Novo Nordisk today announced new data on the investigational oral semaglutide 25 mg will be among 23 abstracts to be presented at ObesityWeek® from 4-7 November in Atlanta, US. The presentations will add to expanding the evidence base on Wegovy®’s overall weight loss efficacy and cardiometabolic profile, and showcase the innovative pipeline with new data for people living with obesity.
“Our diverse range of abstracts reflects the strong momentum of our obesity portfolio and our dedication to improve the lives of people living with obesity. These data reinforce the established efficacy of the semaglutide molecule and highlight the increasing interest within the obesity community in oral options and dosing regimens that may better meet patients’ health needs,” said Martin Holst Lange, chief scientific officer and executive vice president, Research & Development at Novo Nordisk. “This builds on our longstanding track record of prioritizing ObesityWeek® by presenting new science that can make a real difference in patient care. As a pioneer with more than 25 years in obesity, we are proud to again be part of this key event, dedicated to advancing the understanding of obesity as a serious chronic disease.”
OASIS 4 trial highlights include:
Investigating improvements in blood sugar and cardiovascular risk factors
In an OASIS 4 post hoc analysis, investigators explored improvements in glycemic parameters and cardiovascular risk factors for people with obesity or overweight treated with once-daily oral semaglutide 25 mg.1
Examining the efficacy of semaglutide across formulations (pill and injectable) in women’s health
Additional post hoc analyses from OASIS 4 and STEP phase 3a trials examined the efficacy of semaglutide in reducing body weight in women in pre- and post-menopausal stages, and another assessed improved physical function based on patient-reported outcomes data.2,3
Comparing efficacy of semaglutide in a pill with injectable semaglutide (Wegovy®)
Indirect trial comparison of OASIS 4 with STEP 1 comparing efficacy between once-daily oral semaglutide 25 mg with injectable once-weekly semaglutide 2.4 mg.
REDEFINE 1 trial highlights include:
Additional phase three data from REDEFINE 1 included analyses exploring the cardiovascular risk reduction potential of investigational CagriSema and examining the percentage of people treated with CagriSema who achieved clinically relevant treatment targets for obesity management.4,5
STEP UP trial highlights include:
Additional analysis of data from STEP UP with semaglutide 2.4 mg and 7.2 mg assessed risk of obesity-related complications using clinically relevant treatment targets.6
Select Novo Nordisk abstracts to be presented at ObesityWeek ® 2025, 4-7 November [Atlanta, US]:
OASIS 4 (Oral Semaglutide 25 mg)
- Efficacy of Oral Semaglutide 25 mg in People with Overweight or Obesity and Poor Physical Function. Poster presentation – Tuesday 4 November; 7:30-8:30 PM ET
- Improvements in Glycemic Parameters and Cardiovascular Risk Factors with Oral Semaglutide 25 mg. Oral presentation – Wednesday 5 November; 8:15-8:30 AM ET
- Semaglutide Reduces Body Weight Regardless of Menopause Status: STEP and OASIS 4 Post Hoc Analysis. Poster presentation – Wednesday 5 November; 2:30-3:30 PM ET
- Oral vs. Injectable Semaglutide: An Indirect Treatment Comparison of Weight Loss Outcomes. Poster presentation – Wednesday 5 November; 2:30-3:30 PM ET
REDEFINE (CagriSema)
- CagriSema Reduces hsCRP in Adults With Overweight or Obesity: the REDEFINE 1 Trial. Poster presentation – Wednesday 5 November; 2:30-3:30 PM ET
- REDEFINE 5: CagriSema in East Asian Adults With Overweight or Obesity, With or Without T2D. Oral presentation – Wednesday 5 November; 4:00-4:15 PM ET
- CagriSema and achievement of BMI and waist-to-height ratio treatment targets: REDEFINE 1. Poster presentation – Thursday 6 November; 2:30-3:30 PM ET
- CagriSema Reduces Predicted ASCVD Risk in Adults With Overweight or Obesity: the REDEFINE 1 Trial. Poster presentation – Thursday 6 November; 2:30-3:30 PM ET
- Effect of CagriSema 2.4mg/2.4mg on Body Composition, Muscle Strength & Physical Function: REDEFINE 1. Oral presentation – Wednesday 5 November; 3:45-4:00 PM ET
STEP UP (Semaglutide 7.2 mg)
- STEP UP: Final Dose Responder Analysis in participants randomized to semaglutide 7.2 mg. Poster presentation – Thursday 6 November; 2:30-3:30 PM ET
- STEP UP: Efficacy of Semaglutide in Obesity Treatment Targets and Cardiovascular Risk Thresholds. Oral presentation – Wednesday 5 November; 4:30-4:45 PM ET
STEP 9 (Semaglutide 2.4 mg)
- Semaglutide Effect on Physical Functioning in Adults With Obesity and Knee Osteoarthritis. Poster presentation – Tuesday 4 November; 7:30-8:30 PM ET
Semaglutide Real-World Evidence
- Changes in Alcohol/Tobacco Spending Among Users and Non-Users of Semaglutide for Weight Management. Poster presentation – Tuesday 4 November; 7:30-8:30 PM ET
- Factors Related to Adherence and Compliance in Patients With Obesity Receiving Semaglutide. Poster presentation – Tuesday 4 November; 7:30-8:30 PM ET
- Realworld Impact of Semaglutide on Healthcare Utilization and Economic Outcomes in the United States. Oral Presentation – Wednesday 5 November; 8:30-8:45 AM ET
- Lower Real-World Risk of CV Events With Semaglutide 2.4 mg in People with Risk Factors for ASCVD. Oral presentation – Wednesday 5 November; 4:45-5:00 PM ET
Obesity Non-Product
- Association Between Body Mass Index and Surgeries for Knee and Hip Joint Replacements. Poster presentation – Tuesday 4 November; 7:30-8:30 PM ET
Da er nok resultatene ute om max 30 dager
Vel, skal vi titte inn i krystallkula a.k.a The Åtmin Stone?
Oral Sema blir godkjent av FDA. Vi vet allerede at LLYs søknad om å få hurtigavklart orforglipron er avslått, så Oral Sema blir først på markedet. Blir en boost for Novo.
Selv om Ewok’er alltid betyr julestemning, så kommer ikke novo-nissen med godt alzheimernytt. Det store spørsmålet blir hvor mye dette utfallet allerede er priset inn i dagens kurs. Jeg tipper “i stor grad”, noe som trolig betyr “i mindre grad enn det W tror”.
Og hvilken hendelse kommer først?
Novo velger selv å kalle det lottokupong og kjenner jeg bioinvestorer rett så tror nok alle sammen at de skal vinne 1. premie “any day”  (så blir nok greit kursfall dersom nissen kommer med kull)
(så blir nok greit kursfall dersom nissen kommer med kull)
Ja, viktig å huske hvem man eier en aksje sammen med.
Så da er fremdriftsplanen frem til nyttår:
Ned på EVOKE-data
Opp på FDA approval Oral Sema
Ned på LLYs første retatrutide-data
Trolig i den rekkefølgen.
Alt mens kloden spinner rundt og makro nærmer seg “best før”-dato styggfort.
Du gir deg liksom ikke helt med å predikere biologien før markedet har fasiten…
Viking skulle gruse Novo
Lily likeså
Begge kom inn under forventning og i favør av Novo.
Nå skal Novo feile igjen på to av tre forsøk.
Ballsy, men det kan absolutt hende at du får rett. Jeg har i alle fall null peil, så synes det er gøy at noen stikker hodet såpass frem 
Har også forutsett og tatt høyde for 11 av de siste 3 resesjonene 
Following dialogue with the Novo Nordisk Foundation regarding the future composition of the Board of Directors, it has not been possible to reach a common understanding
Chair Helge Lund, Vice Chair Henrik Poulsen and the independent board members Laurence Debroux, Andreas Fibig, Sylvie Grégoire, Christina Law and Martin Mackay will not stand for election at the Extraordinary General Meeting
Item 1.1: Election of chair
It is proposed to elect Lars Rebien Sørensen as Chair of the Board of Directors for a
period until the next Annual General Meeting.Item 1.2: Election of vice chair
It is proposed to elect Cees de Jong as Vice Chair of the Board of Directors for a period
until the next Annual General Meeting.Item 1.3: Election of other members to the Board of Directors
It is proposed to elect Britt Meelby Jensen, Mikael Dolsten and Stephan Engels to the
Board of Directors for a period until the next Annual General Meeting.
Kasim Kutay will continue to serve on the Board of Directors, which is consequently
proposed to consist of six shareholder-elected Board members.
If all proposed candidates are elected, the Board of Directors will consist of Lars
Rebien Sørensen (Chair), Cees de Jong (Vice Chair), Britt Meelby Jensen, Kasim Kutay,
Mikael Dolsten and Stephan Engels (shareholder-elected Board members) and
Elisabeth Dahl Christensen, Liselotte Hyveled, Mette Bøjer Jensen and Thomas
Rantzau (employee-elected Board members).
Se her ja.
Man ser inn i krystallkulen og kommer med (halv)kvalifiserte gjetninger, og så går det bare timer til selveste Novo Nordisk Foundation gjør spåkoneskillsa mine til skamme og bare sender hele dagens styre hjem med kraftig anmerkning i meldingsboka.
Og tidligere CEO gjennom 16 år, Lars Rebius (hvorfor minner navnet meg om en ubåtkaptein?), som kom inn som observatør i styret for NNF i vår (etter at samme NNF kicka ut Fruergaard), foreslås nå som styreleder. Skal NNF ta over hele skuta liksom?
Åpen artikkel i DN: