Dette innlegget ble rapportert og er midlertidig skjult.
Jeg er trolig mest blasert av AI-hypen av alle her inne, men her er det dels tvingende nødvendig at de tar eierskap for hva AI kan (eller mest sannsynlig: ikke kan) gjøre for denne typen billeddiagnostikk.
Dette er jo for å møte konkurransen fra hvitt lys kombinert med AI og som leverer bedre resultater enn hvitt lys uten AI. Om hvitt lys med AI nærmer seg BLC uten AI kan det bli vanskelig å selge inn BLC som jo har en tyngre prosedyre enn hvitt lys. Dette ble diskutert på tråden for ett til to år siden, så det er ikke noe nytt.
AI er jo faktisk veldig bra på bildediagnostikk, noe som er etablert standard innen flere områder. For eksempel har man på enkelte sykehus i Norden erstattet den ene av de to radiologene som gransker bildene med AI siden kliniske forsøk viser at resultatene blir bedre (og man frigjør ressurser/øker kapasiteten).
Det er også gjort forsøk på AI med hvitt lys som viser gode resultater.
Nøkkelen er å ha ett kontrollert datasett av tilstrekkelig størrelse å øve AIen på. Det burde PHO ha, eller skaffe seg fra sine kunder.
Link nederst:
The presentation will be held in English and questions can be submitted throughout the event. The streaming event is available through: https://channel.royalcast.com/landingpage/hegnarmedia/20251029_2/
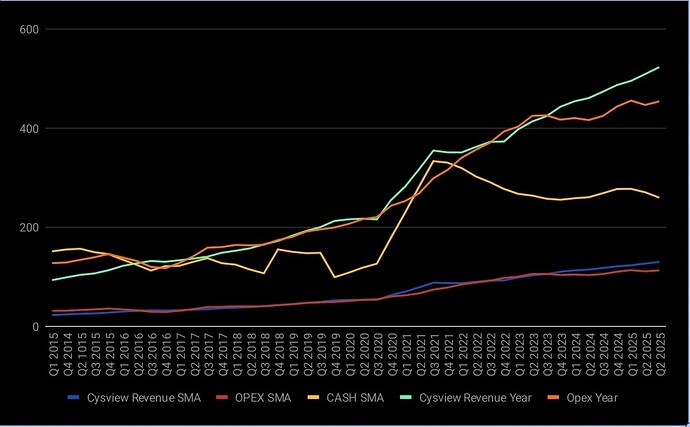
Core business på Cysview vokser, men den vokser veldig sakte.
Her er 4 kvartals glidende snitt, og sum 4 siste kvartaler. Cysview revenue trenger å stige fortere, og opex trenger å holde seg mer eller mindre flatt:
Dette var en svak G+ rapport for Q3, ikke i nærheten av noen M.
Håper de snart bruker nye 50 millioner på tilbakekjøp, ser ingen grunn til at de trenger å sitte på hundrevis av millioner.
Norne
Photocure delivered yet another solid quarter, with 3Q figures broadly exceeding our estimates. The company continued to post double-digit YoY growth on the top line while maintaining well-contained opex. Reported EPS came in above our expectations, and the cash position remains solid. We believe this robust financial performance further underpins our investment case on the stock going forward.
Hypen startet vel i 2018 hvis jeg husker rett. Siden har det gått rimelig trått 
Det kan være et tidsgap mellom økt skop-portefølje og hva som resulterer i løpende salg. Med en stigning på 24% forrige Q og 23% i dette i utplasserte skop, noe som er svært positivt, mens det er en salgsøkning på 14 % på ett år bør det ligge an til en implisitt salgsøkning av Cysview fremover. Avgjørende er frekvensen i bruken. Det blir vel litt tilbakeskuende i din vurdering. Det positive nå er at Pho hele tiden bedre sine salgsbase.
ForTec has acquired and deployed 6 additional BLC towers, bringing the total number of mobile BLC towers to 24. With the increasing momentum provided by ForTec’s mobile solution, Photocure had 373 active accounts in the U.S. at the end of the quarter, an increase of 23% versus the second quarter of 2024.
Ser du på plottet egentlig? Var jo en periode rett før corona veksten var eksplosiv, så flatet det helt ut igjen. Er sånn trend de trenger igjen, uten at kostnadene stiger særlig.
Det kan være grunn til å legge vekt på de senere NMIBC-rapportene og at selskapet mener det vil gi vedvarende inntektsvekst.
DS skriver: Looking ahead, we anticipate sustained revenue growth, fueled by rigid kit adoption, expansion of mobile BLC, and HD upgrades that enhance utilization and sales. The tailwinds from a wave of recently approved NMIBC therapeutics are raising awareness around early detection and personalized disease management, validating Photocure’s position at the center of this rapidly evolving ecosystem.
New budget impact model study in 4 European countries concludes that BLC use offers a clinically meaningful and economically rational approach to NMIBC management
Photocure receives prestigious Innovation Prize from the Norwegian Cancer Society: Recognition for Groundbreaking Contributions in Bladder Cancer Diagnostics
The impact of avoiding recurrence: New BRAVO Study abstract at SUO 2025 demonstrates Cost Neutrality in Blue Light versus White Light Cystoscopy comparison
New publication: examining 12-months recurrence-free survival following photo coagulation of bladder tumor versus TUR-BT
Photocure announces the publication of the study “In-office laser coagulation of Ta bladder tumor compared to TUR-BT: 12 months follow-up randomized clinical trial” in the Journal of Urology last week. A first abstract of the study’s 12-month data had been presented at the European Association of Urology (EAU) 2024 congress. Photocure has supported this program and the specific study since 2016.
Laser III is a prospective, randomized, non-inferiority trial conducted in Denmark (NCT02886026) aimed to determine whether in-office photo coagulation bladder tumor (PC-BT) is non-inferior to standard TUR-BT regarding 12-months recurrence-free survival (RFS) in patients with recurrent Ta low grade bladder tumor. Both the office-based laser procedure and TUR-BT procedure were performed under BLC-guidance, comparing the procedures with the most complete detection technology and without compromising clinical or oncological safety. From 2016 to 2022 a randomized controlled clinical trial was performed comparing PC-BT with 980 nm diode laser under local anesthesia with gold standard TUR-BT under general anesthesia, in 300 patients in a hospital setting in Denmark.
Study results show 12 months recurrence-free survival was 43.5% after PC-BT and 43.0% after TUR-BT, the difference 0.5% in favor of TUR-BT met the noninferiority criterion.
The authors conclude: “Office-based laser photo coagulation of bladder tumor is non-inferior to TUR-BT regarding 12-months recurrence-free survival and is a safe, efficient treatment for recurrent Ta low-grade bladder tumors”
“ This is another quality clinical study supporting the utility of BLC for improving surgical performance, by enhancing detection of tumors and visibility of tumor margins, and its impact on clinical outcomes ,” said Anders Neijber Chief Medical Officer of Photocure.