Det virker jo som om noen har satt seg ett mål om at rep emi ikke skal gjennomføres, så å legge seg på 55.5kr er kanskje ikke det dummeste i verden. Men, jeg syntes også at å kjøpe rett rundt 60kr er relativt god risk/reward - selv om det for min del da er gambling på at selskapet skal ha noen positive nyheter i løpet av kort tid.
ja, føler på det samme, kjøper nå selv om den kan falle litt til.
Klart der er en XX kroner å spare på 5kr ned , men problemet er nå at denne gangen skal eg være med opp  så da må en inn
så da må en inn 
Blir fort interessant nå 
Har vært åpenbart lenge nå. Mon tro hvor mange ganger de doble volumlokket på ask må tømmes før vedkommende finner ut at nok er nok. Enn så lenge er det ingen med litt muskler som har giddet å finne ut av det. Personlig synes jeg det er på overtid.
kan nå pirke litt 
Er jo ikke tenkelig at det skjer at rep emi kanselleres.
(vii) Tegningsperioden er fra og med 2. februar 2021 til og med 16. februar 2021. Start av tegningsperioden er avhengig av ferdigstillelse og publisering av Selskapets nasjonale tilbudsprospekt innen dette tidspunktet.
Forhåpentligvis kommer prospekt i morgen.
Ved eventuell forsinkelse av prospektet vil tegningsperioden (og datoene henvist til i dette punktet) bli utsatt tilsvarende etter styrets nærmere beslutning). De nye aksjene gir rett til utbytte fra den dato kapitalforhøyelsen registreres i Foretaksregisteret. (viii) De nye aksjene tegnes på et særskilt tegningsformular. (ix) Innbetaling av tegningsbeløpet skal skje senest 28. februar 2021 til særskilt emisjonskonto, som nærmere angitt av SpareBank 1 Markets AS.
Noen har vist oppdatert Softox på Reddit også.
For 4 måneder siden med null interesse ja 
Les litt lengre ned, lagt inn linker også 
Jeg er tydeligvis blind, ser ikke hva du refererer til ?
Ser bare to poster som er skrevet/redigert for 4 måneder siden ?
hmm…jeg ser (da jeg har skrevet) men kanskje ikke godkjent enda?
SoftOx launches fast-track inhalation solution studies for the treatment of COVID-19 patientsand claims the antibiotic is a breakthrough for mutated viruses.
EXCITE International is a global collaboration of key stakeholders. EXCITE and Blue Cross/ Blue Shield recommended SoftOx to MTEC and US Navy.
This recommendation has given Softox this on SWIS-01:
US Department of Defense (DoD) awarded SoftOx $1.977 million (USD) for research and development of the SoftOx Infection remover (Biofilm Eradicator). NMRC and SoftOx have weekly meetings, monthly reporting, and are active partners in the clinical development
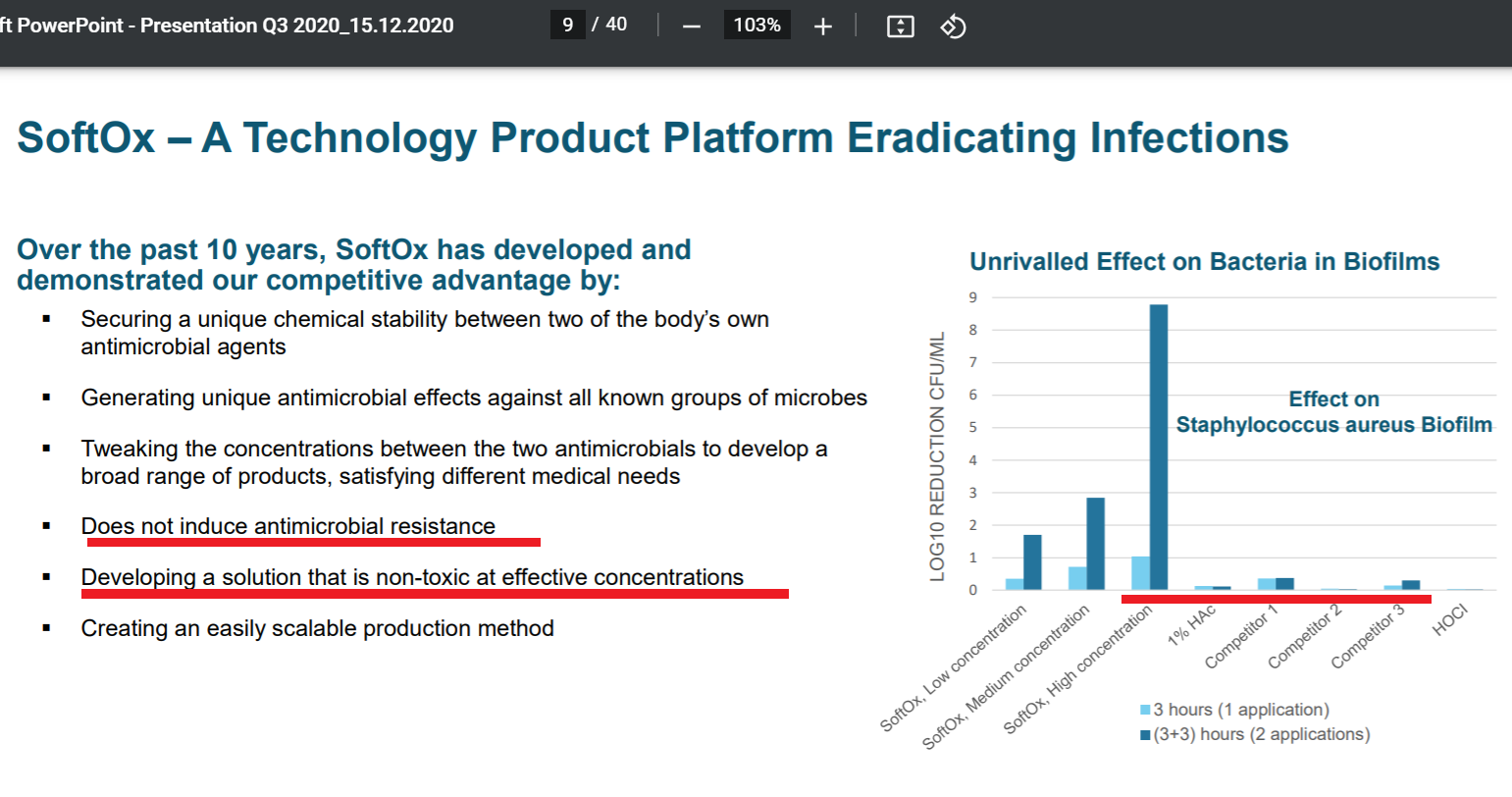
Graf Biofilm:
https://images.app.goo.gl/BMFux9A6Ztncjqwu5
https://newsweb.oslobors.no/message/517037
Softox SWIS-02 Wound healer is expected finish May/jun 2021 and will get 510(k) in US so its hopefully in the market late 2021.
Softox is also in WHO with handdesinfection POPS and has hope WHO will recommend this handdesinfetion against resistant bacteria.
https://www.who.int/initiatives/private-organizations-for-patient-safety/pops-for-hand-hygiene
Softox products have potensial of a very huge market and have today a market price around 500 million Nok.
Potensial BIG rocket…
https://live.euronext.com/en/product/equities/NO0010811961-MERK
“noen” har oppdatert 


Det er ett bra tiltak å spre informasjon om selskapet rundt omkring, men kanskje greit å si at du er forfatter 
Begynner å summere det de antyder inhalator, SWIS, desinfeksjon ++(alt annet det kan virke mot) nærmer vi oss minst 300Mrd med alt og kursen står til 1/600 del av dette. Alt dette er nesten for godt til å være sant, hvis de lykkes.
Mulighetene for å lykkes kan hvert fall ikke bli bedre enn forskningsresultater som viser uovertrufne egenskaper. Vel og merke i tidlig fase. Langt til mål, men dette er jo ikke som i biotek hvor det overhodet ikke er en garanti for at resultater i et forsøk på mus er overførbart til mennesker. Vi snakker minigris, som er tilnærmet identisk med mennesker.
I mitt hode blir største bøygen markedspenetrasjon. Overbevisningen om at produktene (minus inhalatoren som er et longshot) har livets rett er skyhøy. Spørsmålet om noen år blir om de lykkes i stor nok grad med å kapre markedsandeler og i hvilket tempo. Som er hvorfor det er gull verdt at selskapet får øvd seg og trukket erfaringer på desinfeksjonsbiten
Jeg tipper at når SWIS er ferdig med siste studie og klar for markedet + BE og SIS på fase 1 så begynner noen å snuse på å kjøpe selskapet.
Sannelig har ikke “noen” lagt ut noe om Softox på Yahoo også… skal lete om det også kan finnes andre steder snart 
https://finance.yahoo.com/quote/SOFTX.OL/community?p=SOFTX.OL
Tror det nærmer seg en slik tvihold på aksjer periode.
I allefall ikke lurt å ligge i boken på selgersiden om der kommer noe melding , da er du brått i fomo land uten aksjer