Ingen salg i Europa? Med godkjenning 15Juli og partner som var klar burde ikke salget være i gang eller tar dette bare lengre tid?
Prisene for Tyskland kom vel for ca 1 mnd. siden så da burde det blir salg i Tyskland og milestone utbetaling i Q4?
Hva tenker vi om noenlunde likt antall enrollments i Q3 som i Q2 - omsetning vil fortsatt øke som følge av at betaling er fordelt over et år og med forsinkelse på betaling i forhold til “enrollment”.
Det vil være utrolig viktig hva Renee sier i presentasjonen om nye enrollments og status rundt breakeven. Det kan avgjøre hele dagen i dag.
I rapporten står det noe om “strong september month” som da gir en viss pekepinn på hvordan ordlyden kommer til å være i dag. Fokus mot neste Q tror jeg.
Der er kommet Salg i DE i Oktober allerede - dog kun en 10-20 pakker. Ved dog heller ikke hvor længe de havde mulighed for at sælge i Oktober (i.e. Hele måneden eller kun et par dage). Er ved at undersøge hvorvidt der allerede er træk på Unlicensed Medicine markedet i Europa, for de lande hvor det vil tage længere tid for STADA at få produktet lanceret. Det skulle gerne give en god indikation hvor ivrige læger/patienter er efter at få fingrene i medicinen.
Calliditas Q3 outlook
2022-11-14
09:45
Calliditas recorded Q3 product sales of SEK 125m in Q3 which is very close our and the markets expectations. The number of unique (US) subscribers has increased by >50% in Q3 and Calliditas will guide for the full year sales at the approaching Q4 report. Together with the milestone related SEK 135m income from Calliditas European partner STADA as a result of the European approval this is another strong quarter from Calliditas.
Johan Unnerus
The expanded sales force is already in place.
Calliditas recorded Q3 product sales of SEK 125m in Q3, which is very close to our and the market’s expectations. The number of unique (US) subscribers has increased by >50% in Q3, and Calliditas will guide the full-year sales in the approaching Q4 report. Together with the milestone related SEK 135m income from Calliditas European partner STADA as a result of the European approval, this is another strong quarter from Calliditas.
The Sales are increasing faster than the increase in the number of unique subscribers, which is probably a result of the seasonally softer Q3 period, including the holiday period. Callidita’s outlook for the entire year of Tarpeyo sales of USD 35-40 suggests a Q4 sales of SEK 155-210m, slightly subject to USD/SEK changes. This is just below our and the market expectations for Q4 product sales, where we expect SEK 215m of sales.
Calliditas will guide the 2023 sales when reporting the Q4 results on the 23rd of February, 2023. Market expectations are product sales of SEK 1.9bn, and we also expect SEK 1.9bn in product sales for 2023. The table below shows the Q3 outcome:
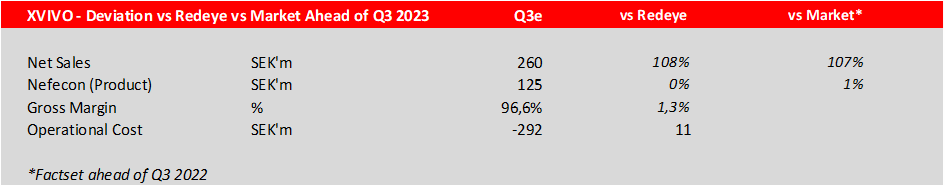
The gross profit related to product sales is a robust 96,5%, slightly better than we expected which is reassuring. The operational expenses increased to SEK 292m, which was just below our expected SEK 302m. This can be expected to rise during Q4 due to R&D activities and the expanded US sales force from 40 to 60 sales representatives that are now in place.
We expect a muted market reaction on the back of the Q3 result. At face value, the sales outlook for Q4 can be considered modest, but it could also be set rather conservatively at this early stage of the launch. Callidita’s ability to increase the number of unique US subscribers backed by the support of the expanded sales force will be important during Q4, especially when Calliditas has already established a higher and robust level of commercial reimbursement cover with corresponding vital dynamics in terms of a timely conversion from unique subscribers into deliveries and recorded sales.
Structurally 2023 will provides some fundamental answers to critical questions. How extensively will Nefecon be used and prescribed among specialists, to what extent will NefIgArd Part B results (H1 2023) show Tarpeyo’s ability to stabilize eGFR after 12m and when will Tarpeyo face some respectable competition?
Additional clinical eGFR support will likely convert the approval into unconditional approval and extend the market lead over future potential competition. Remember that reduced proteinuria is a well-accepted and established marker, but clinically the critical objective is to protect and stabilize the eGFR filtration for this patient group. In the second part of the NeflgArd study (Part B), eGFR filtration is the primary endpoint, not proteinuria (the reverse vs Part A). This is now even more interesting when the potential competition to Tarpeyo has struggled to produce sufficient clinical evidence in this space earlier this summer.
We regard the current valuation as distinctly undemanding, with a significant upside ahead. Of course, the fundamental value proposition is still obscured by macroeconomic and geopolitical issues with no direct connection to Calliditas. Our fundamental value proposition is a base case value of SEK 280 (Bull 450, Bear 125) and Calliditas now trades substantially below our Bear case.
CALLIDITAS THERAPEUTICS
Everest Medicines regulatoriska ansökan om marknadsgodkännande för Nefecon accepterat av kinesiska myndigheten NMPA (Cision)
2022-11-15 08:00
Calliditas Therapeutics AB (Nasdaq: CALT, Nasdaq Stockholm: CALTX) (“Calliditas”) meddelade idag att den kinesiska myndigheten National Medical Products Administration (NMPA) har accepterat Everest Medicines (HKEX 1952.HK, “Everest”) regulatoriska ansökan om marknadsgodkännande (New Drug Application, NDA) för Nefecon. Bifallet av ansökan för Nefecon, som är godkänd och marknadsförd i USA under namnet TARPEYO[®] och i EU som Kinpeygo[®], för Nefecon ett viktigt steg närmare att potentiellt bli det första godkända läkemedlet för behandling av primär IgA-nefropati i Kina.
I december 2020 rekommenderade NMPA s k Breakthrough Therapy Designation (BTD) för Nefecon för behandling av IgAN. Kronisk njursjukdom är ett av de allvarligaste folkhälsoproblemen i Kina, där IgAN enligt Everest beräknas drabba cirka fem miljoner människor.
"Det är spännande att Everests NDA har accepterats av NMPA, vilket skapar en väg framåt för att kunna hjälpa den betydande patientpopulationen som lider av IgAN i Kina. Vi ser fram emot att fortsätta vårt samarbete som fokuserar på att utveckla innovativa behandlingar för patienter”, säger VD Renée Aguiar-Lucander.
I juni 2019 ingick Calliditas ett licensavtal med Everest för att utveckla och kommersialisera Nefecon i kinesiska regionen och Singapore i IgAN. Detta avtal utökades till att omfatta Sydkorea i mars 2022.

可以是六个月吗?
spurte på discorden hvor lang behandlingtid de forventet i kinesisk legemiddelverk
fikk til svar: 可以是六个月吗?
 (den var lett å googletranslate da)
(den var lett å googletranslate da)
Mon tro om dette er det lille nudget som kan dytte oss nord for hundrelappen, jeg regner med alt av tradere er ute i dag? 
huh, skal du kaste meg ut? 
Hæh?
Skal du ut?
Tradere kom seg vel ut i går etter en høvelig nøytral dag…
PAS:
Nefecon’s NDA accepted in China
Calliditas Therapeutics today announced that the Chinese regulatory authority, the National Medical Products Agency (NMPA) has accepted the new drug application (NDA) of Nefecon by Calliditas’ Asia partner Everest Medicines. We expect regulatory approval in China in the second half of 2023. Nefecon would be, like in the US and Europe, the first approved drug in China for IgA Nephropathy, where the addressable market includes approximately five million patients. For now, we have not valued China and plan to do so after launch within the region. Either way, this just adds additional upside. With (i) positive additional clinical data out, (ii) negative developments for the still development-stage competition and (iii) growing sales we continue to see CALTX as highly undervalued. We reiterate our Buy rating on CALTX with a target price of SEK 300.
En populasjon på 5 millioner med IgA i Kina er noe å gripe fatt i  !
!
En het oppkjøpskandidat ved FDA godkjennelse vil jeg tro! 
Kan man forvente en endelig godkjenning av FDA i løpet av 2023?
Nei…tipper engang i første halvår 24
Fin og lærerik artikkel om IgA nefropati i Tidsskriftet. Budesonid er nevnt:
«Nærmest klinisk bruk i Norge er kanskje budesonid (24), som er foreslått til vurdering i systemet Nye metoder. Resultatene fra en fase 3-studie er ventet i 2023.»



