Problemet er hva er det de skal markedsføre?
Tror de står fritt til å bare kalle det Semaglutide API
Så forresten på Bloomberg her at Novo planlegger å søke FDA om godkjenning av 7,2 mg sema (3x godkjent dose i dag). Martin Lange sa i går noe sånn som at “de andre selskapene [les: LLY] har optimert sine doser, det gjør vi nå også”. 7.2mg-dosen av Semaglutid vil nesten helt garantert klarere FDA. Og greia er at når den gjør det, så sitter NVO med en subkutant levert legemiddel, som egentlig mer eller mindre matcher tirzepatide i efficacy og tolererbarhet.
De lave dosene som compounders tilbyr tror jeg vil bli utkonkurrert av billige piller som orforglipron. Tipper prisene kommer til å matche ganske greit. Vært inne på temaet før, altså “turistklassen” i fedmemarkedet. Lave doser sc vil kanskje ha noe bedre tolererbarhet enn piller, men efficacy vil ikke være bedre.
Men de kan ikke referere til hva effekten er? Siden deres versjon ikke har blitt kjørt gjennom FDA-testingen?
Altså, for å forklare hva greia er med ikke-peptide small molecules: Dette er fra callen til Structure Therapeutics (GPCR) hos Morgan Stanleys 23rd Annual Global Healthcare Conference:
“With small molecules, right now today at Structure Therapeutics, we have the ability to make 6,000 metric tons. What is – what exactly does that mean in terms of patients? We can make enough material today to supply the needs of 100 million patients at 120-milligram dose.”
GPCR er et “jallabio” med MCAP på 1,1 millard USD. Legemidlet de har som er lengst fremme i pipe er aleniglipron som altså er i et par fase IIb’er som leser av nå i q4. Så kan man jo bare tenke seg hva produksjonskapasitet hos en gigant som LLY for orforglipron faktisk må være.
NVO gjennomførte en spkalt “fireside chat” ved EASD i går. Ennå ikke noe tilgjengelig transcript fra den, men noen slides har blitt lagt ut. Sakser ut den kanskje mest vesentlige av dem alle:
Litt rar header på sliden. Burde kanskje ikke stå bare “2026”, men kanskje “litt inn i 2026”. Punkt 1, 2, 4, 5 + fase III-resultat i Alzheimers er ihvertfall guidet 2025, selv om siste kanskje kan gli inn tidlig 2026. Er noe add-on greier på de trialene, så selv om de egentlig avsluttes nå i september, så skulle pasienter følges ut oktober elns.
REDEFINE-4 er h2h CagriSema vs. Tirzepatide. Etter hva jeg kan finne så er den uten den jalla flexiprotokollen som REDEFINE-1 hadde. CagriSema har fått et dårligere rykte enn fortjent (pga idiotisk guiding fra NVO på efficacy), og burde ihvertfall klare målet om “non-inferiority”. Jeg tipper resultatet vil ende på 2-2,5% bedre efficacy enn tirzepatide. Så blir det store spørsmålet når alle pas. er “tvunget” til å ta 2.4 mg (cagri) og 2.4mg (sema), altså maksdosen fra REDEFINE-1, hvordan blir tolerability? Mener REDEFINE-4 “egentlig” hadde trial completion i august 2025, så data derfra kan komme ramlende fortere enn man tror.
Firesidechat’en kan høres her:
https://edge.media-server.com/mmc/p/boyxgi4o
Denne kom også igår kveld:
Novo Nordisk’s oral semaglutide 25 mg (Wegovy® in a pill*) delivered 16.6% weight loss in people with obesity in a newly published study
- Oral semaglutide 25 mg (the once-daily pill formulation of Wegovy®) achieved significant weight loss, with one in three study participants losing 20% or more body weight1**
- Oral semaglutide 25 mg also demonstrated improvements in the ability to perform everyday physical activities such as bending, standing, walking, being physically active, and improvements in cardiovascular risk factors1
- Oral semaglutide 25 mg is the first oral GLP-1 therapy submitted to the US Food and Drug Administration (FDA) for chronic weight management,2 and Novo Nordisk has already begun production at its US sites
Bagsværd, Denmark, Plainsboro, NJ, 17 September 2025 – Today, The New England Journal of Medicine published the results from the OASIS 4 phase 3 trial that studied the efficacy and safety of the investigational once-daily oral semaglutide 25 mg (Wegovy ® in a pill*), marking a significant milestone in the company’s ambition to advance obesity care. In the 64-week trial, oral semaglutide 25 mg, alongside lifestyle modifications, was compared to placebo in 307 adults with obesity or overweight with one or more weight-related comorbidities, without diabetes.1
Results showed that if all participants adhered to treatment, average weight loss of 16.6% was achieved by people taking oral semaglutide 25 mg compared to 2.7% for placebo at 64 weeks, with over a third (34.4%) experiencing a weight loss of 20% or more, versus 2.9% for placebo.1**
This was comparable with previous trial results of injectable Wegovy®.3When looking at the effect regardless of whether the participants took the medicine exactly as they should, people taking oral semaglutide 25 mg still achieved an average weight loss of 13.6% versus 2.2% with the placebo.1*** Close to a third (29.7%) had a weight loss of 20% or more versus 3.3% for placebo.1*** Additionally, the study also found that oral semaglutide 25 mg improved cardiovascular risk factors, as well as the ability to be more active in daily life, compared to placebo. This was consistent with previous trial results of injectable Wegovy®.1,3
“The oral semaglutide 25 mg data show compelling efficacy for an oral weight management medication with 16.6% weight loss and a safety and tolerability profile consistent with injectable Wegovy®,” said Martin Holst Lange, chief scientific officer and executive vice president of Research & Development at Novo Nordisk. “Currently, less than 2% of individuals with obesity in the US receive obesity medication and Wegovy® in a pill may also address patient preference for oral treatment. Pending FDA approval, ample supply will be available to meet the expected US demand as we hope to set a new treatment benchmark for oral weight loss medications for people with overweight or obesity.”
The safety and tolerability profile of oral semaglutide was consistent with that for injectable Wegovy®.1,3 In the OASIS 4 trial, gastrointestinal adverse events with oral semaglutide 25 mg were generally mild to moderate in severity and transient; the most common of which were nausea (46.6% versus 18.6% for placebo) and vomiting (30.9% versus 5.9% for placebo).1 Adverse events leading to permanent treatment discontinuation were 6.9% (oral semaglutide 25 mg) and 5.9% (placebo). The incidence of serious adverse events was 3.9% (oral semaglutide 25 mg) and 8.8% (placebo) . 1 This reinforces the safety and tolerability profile of semaglutide, as also demonstrated in more than 37 million patient-years of exposure.1,4
“The OASIS 4 trial results further underscore the significant impact that semaglutide can have in achieving sustainable weight loss and broader health benefits,” said Sean Wharton, lead study author and medical director of the Wharton Medical Clinic. “Oral semaglutide 25 mg builds on the proven efficacy and established safety and tolerability profile of semaglutide and represents a significant advancement in obesity treatment. People with overweight or obesity have individual preferences, and with oral semaglutide as a potential new treatment option, more of those who are not on treatment today may consider starting GLP-1 treatment.”
In February, Novo Nordisk submitted a New Drug Application (NDA) for the once-daily pill formulation of Wegovy®.2* The FDA review of this NDA is anticipated to be completed by the end of this year.5 Currently, there are no approved oral formulations of a GLP-1 medicine for weight loss.
If approved by the FDA, the pill for weight management will be fully made in the US, with production already underway at Novo Nordisk’s significantly expanded manufacturing facility.
Lukter “the ultimate comeback kid” av Novo om dagen, det meste går i deres favør.
Novo Nordisk A/S: Ozempic® reduces the risk of heart attack, stroke and death by 23% compared to dulaglutide in the first head-to-head real-world study
- Ozempic® (once-weekly injectable semaglutide) was associated with a 23% reduced risk of heart attack, stroke and death in people with type 2 diabetes and cardiovascular disease on Medicare versus dulaglutide1
- Data also highlighted Ozempic® was associated with a 26% lower risk of death versus dulaglutide1
- This study is the first to directly compare these glucagon-like peptide 1 receptor agonist (GLP-1 RA) medications in everyday real-life use. It fills a significant gap in understanding their effects on heart health in older people at high risk, which could help support informed treatment decisions and health policies.1
Bagsværd, Denmark, 18 September 2025 – Novo Nordisk today announced results from the REACH real-world study, which demonstrated that compared to dulaglutide, Ozempic® (once-weekly injectable semaglutide) was associated with a reduced risk of major adverse cardiovascular events such as a heart attack or stroke by 23%1. The data span nearly 60,000 US Medicare patients (aged ≥66 years) living with type 2 diabetes, atherosclerotic cardiovascular disease (ASCVD) – a condition where fatty deposits build up in blood vessels, reducing blood flow and increasing the risk of heart attacks, strokes and related problems – and multiple health conditions2. The results were presented at the European Association for the Study of Diabetes (EASD) 2025 Annual Meeting on 15–19 September in Vienna, Austria1.
“As we age, the risk of experiencing a heart attack, stroke or dying from a cardiovascular event increases. At the same time, there are limited clinical data for people living with diabetes and cardiovascular disease aged 66 years or older. These data, showing a 23% risk reduction of a heart attack, stroke and death, fill an important gap and reinforce the well-established clinical evidence of semaglutide,” said Filip Krag Knop, senior vice president and incoming chief medical officer at Novo Nordisk. “This is great news for older patients as well as healthcare professionals, as these results build on the importance of our randomised clinical trial data assessing the effectiveness of treatments in a real-life setting. This also supports what we already know from our clinical development programmes that not all GLP-1 RAs are the same.”
Beyond these essential benefits, once-weekly semaglutide was also associated with a 25% risk reduction of heart attack, stroke, hospitalisation for unstable angina or heart failure, and death from any cause (five-point MACE)1.
Ozempic® is the only GLP-1 RA that has proven risk reduction of cardiovascular and kidney events in people with type 2 diabetes3-6. These results provide the first direct comparison of cardiovascular outcomes between Ozempic® and dulaglutide in US Medicare beneficiaries and add to the body of evidence for Ozempic®.
29/9 deadlinen til DJT og “Most Favoured Nations”-opplegget hans vedr. medisinpriser nærmer seg btw. Risikoavers som jeg er har jeg skalert litt (midlertidig) ned, selv om jeg samtidig antar at Novos internasjonale tilfang innebærer at selskapet ikke blir det mest skadelidende BP’et om det skulle bli en del støy rundt dette.
Rundt 30% avkastning på nesten null tid må jo være bra risk/reward. 
Litt irriterende å ha så lite overbevisning som jeg hadde som førte til for tight stop loss slik at jeg gikk glipp av dette. Ikke overrasket over at kursen her fikk seg en rekyl, etter diskusjonen til @TheObserver og @WernerVonHaussenberg. 
Som vi ser fra fireside chat’en som jeg og @theroger lenket til over her, så kommer en del nyheter fra NVO fremover. Mange av dem vil trolig være ganske gode, og markedet vil nok reagere bra på dem. Men først skal det kanskje bli litt baluba med hele det medisinprisingsopplegget til den amerikanske presidenten. Like greit å sitte for det meste utenfor på kort sikt, tror jeg.
Korrekt - men tillige skal stadig huskes, at NOVO er kraftigt repræsenteret med fabrikker/ansatte m.m. i USA.
Jf. AI
Novo Nordisk driver flere end tre produktioner i USA, hvilket omfatter en nyinvesteret fabrik i Clayton, North Carolina, og to andre. Det præcise antal fabrikker er ikke angivet, men det oplyses på deres amerikanske hjemmeside, at de har faciliteter i 12 forskellige lokationer i USA, hvilket inkluderer både firma- og forskningsfaciliteter.
Novo Nordisk har over 10.000 medarbejdere i USA efter en stor investering i nye fabrikker i begyndelsen af 2025. Selvom der tidligere var oplyst, at virksomheden havde cirka 2.500 ansatte i North Carolina alene, indikerer nyere tal en markant stigning i medarbejderantallet i USA generelt.
Ikke minst dette utsagnet
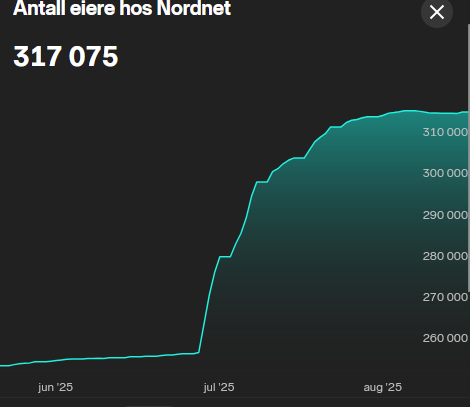
Men viktig å tenke på at her er det kommet inn en drøss nye “investorer” i det siste og aksjen er voltatil
Han kan vi hvert fall ikke prosentregning.
Prisene kan jo bare holdes stabile i nominell dollar, og så lar vi det stabile genient lage inflasjon av typen Weimar eller Zimbabwe?
Nope. Men man kan jo håpe at det tar slutt når fristen går ut om 6 dager.
Nedgang i vekt og midjemål
Deltakerne fikk ukentlige sprøyter i 72 uker. 1005 deltakere fikk 7,2 milligram av slankemedisinen Wegovy, der virkestoffet er semaglutid. 201 personer fikk dagens standarddose på 2,4 milligram, og 201 fikk narremedisin.
Alle deltagere fikk kostholdsveiledning og anbefalinger om økt fysisk aktivitet.
Etter testperioden hadde de som fikk trippeldose, i snitt gått ned 19 prosent i vekt, mens de som fikk standarddosen hadde gått ned 16 prosent. De som sto på narremedisinen hadde gått ned 4 prosent i vekt.
En tredel av de som fikk trippeldosen, hadde gått ned 25 prosent eller mer i vekt.
Spot on call