Ingen stor påvirkning på kursen enda…dessverre.
Denne preprint-saken ble publisert i dag, bemcentinib/r428 nevnes et femtitalls ganger. Det var en interessant artikkel og gøy at bemcentinib også får oppmerksomhet i forbindelse med eggstokkreft. Jeg har bare skumlest artikkelen, men så langt ser den veldig bull ut for bemcentinib.
Når det er sagt stusser jeg over hvordan det er mulig gjøre den feil å skrive flere ganger at bemcentinib allerede er godkjent for AML av FDA, to avsnitt som viser dette:
The first-inclass AXL inhibitor bemcentinib (R428/BGB324) was approved for treatment of acute myeloid leukaemia and was also reported to show selective sensitivity towards ovarian cancers (OC) with a Mesenchymal molecular subtype.
Recently, the first-in-class AXL inhibitor bemcentinib (R428/BGB324) was approved by the FDA for the treatment of relapsed or refractory acute myeloid leukaemia [11, 12]. Clinical trials focusing on combination regimes between AXL inhibition and chemotherapeutic agents, or immunotherapies are being launched. Therefore, uncovering novel combination strategy with AXL remains critical.
Den eneste forklaringen jeg ser, er at de egentlig sikter til fasttrack for AML som bemcentinib fikk? Jeg går i surr med hvor mange faststrack de har nå, er det ikke tre stykker? Én for AML og to for NSCLC/STK11?
Oppdatering: Jeg letet, og det er tre stykker (jeg trodde det kanskje var fire, men husket feil): Oktober 2019 AML, juni 2021 ANTI-PD-(L)1/NSCLC og november 2021 STK11/NSCLC
The resistance studies come on the heels of other recent concerns about Paxlovid… (klipp) Confirming anecdotal reports widely reported by media, several studies have found a small percentage of infected people who receive the normal 5-day course initially feel better, only to have their symptoms rebound. And questions have grown about whether Paxlovid helps those who aren’t at high risk of serious disease — Pfizer earlier this month halted a large trial of the drug in standard risk COVID-19 patients because it was failing to show statistically significant protection against death or hospitalization.
Neimen hoppsann, er ikke Pax. så fantastisk allikevel som det ble hevdet her inne tidligere?!
Synes det er litt rart egentlig ang. disse medisinene. Covid fungerer på to måter: Man får viruset og immunforsvaret tar livet av det ila. 1-3 dager hos nesten alle. Så går noens immunforsvar inn i en cytokinstorm, og det er her Bemcentinib fungerer i å dempe immunresponsen ETTER at coviden er ute av kroppen. Skjønner ikke at det ikke trekkes frem mer. Det er cytokinstormen som er det farlige med covid, at kroppen gjennom immunresponsen angriper seg selv. Cytokine storm - Wikipedia Covid har noen andre effekter, men respirasjonsproblemene og det meste annet dødelig kommer av cytokinstormen, ikke Covid i seg selv.
Dagsfersk bulling fra forskere om bemcentinib/r428. Er så fersk at DOI ikke fungerer enda: https://doi.org/10.3390/cells11132078 Denne var ekstra artig: “It is promising that R428 may be approved for clinical use in the future.” 
Abstract
Integrin ß3 plays a key role in the resistance to epidermal growth factor receptor tyrosine kinase inhibitors (EGFR-TKI), but the development of integrin ß3 inhibitors has been stalled due to the failure of phase III clinical trials for cancer treatment. Therefore, it is imperative to find a potentially effective solution to the problem of acquired resistance to EGFR-TKI for patients with integrin-ß3 positive non-small-cell lung cancer (NSCLC) by exploring novel downstream targets and action mechanisms of integrin ß3. In the present study, we observed that the expression of integrin ß3 and AXL was significantly upregulated in erlotinib-resistant NSCLC cell lines, which was further confirmed clinically in tumor specimens from patients with NSCLC who developed acquired resistance to erlotinib. Through ectopic expression or knockdown, we found that AXL expression was positively regulated by integrin ß3. In addition, integrin ß3 promoted erlotinib resistance in NSCLC cells by upregulating AXL expression. Furthermore, the YAP pathway, rather than pathways associated with ERK or AKT, was involved in the regulation of AXL by integrin ß3. To investigate the clinical significance of this finding, the current well-known AXL inhibitor R428 was tested, demonstrating that R428 significantly inhibited resistance to erlotinib, colony formation, epithelial–mesenchymal transformation and cell migration induced by integrin ß3. In conclusion, integrin ß3 could promote resistance to EGFR-TKI in NSCLC by upregulating the expression of AXL through the YAP pathway. Patients with advanced NSCLC, who are positive for integrin ß3, might benefit from a combination of AXL inhibitors and EGFR-TKI therapy.
3.5. AXL Inhibition Reverses Erlotinib Resistance and Colony Formation Induced by Integrin ß3 in NSCLC Cells
Given the involvement of AXL in integrin ß3-mediated erlotinib resistance, the well-known AXL inhibitor R428 was tested. As a highly selective single-target inhibitor, R428 targets AXL in an ATP-competitive manner. Previous studies have shown that R428 reverses acquired resistance to erlotinib in patients with triple-negative breast cancer [22]. In order to investigate the effect of R428 on acquired resistance to erlotinib induced by ectopic expression of integrin ß3, the transfected cells were treated with erlotinib at different concentrations. As expected, R428 significantly reversed the increased survival of HCC827 and HCC4006 cells upon erlotinib treatment induced by ectopic expression of integrin ß3 (Figure 6A,B). Moreover, overexpression of integrin ß3 promoted colony formation, which could be reversed by R428 in NSCLC cells (Figure 6C,D).
3.6. AXL Inhibition Reverses EMT and Migration Induced by Integrin ß3 in NSCLC Cells
EMT promotes resistance to targeted therapies and chemotherapeutic agents, which may be an indirect but major mechanism of drug resistance. EMT has been reported to be associated with acquired resistance to EGFR-TKI [23]. We hence explored the effect of AXL on EMT induced by integrin ß3. As expected, ectopic expression of integrin ß3 induced vimentin expression and reduced E-cadherin expression, effects that were reversed by R428 (Figure 7A,B). In addition, R428 could suppress cell migration promoted by the overexpression of integrin ß3 (Figure 7C,D). These results suggested that AXL inhibition could reverse EMT and cell migration induced by integrin ß3 in NSCLC cells.
By investigating the mechanism of EGFR-TKI resistance induced by integrin ß3, we aimed to provide an alternative strategy to obviate to the current situation of failed clinical trials of integrin ß3 inhibitors. Due to the high selectivity of R428, induction of apoptosis in the absence of AXL is limited, which provides R428 with the potential advantage of causing less side effects in cancer therapy. Phase I/II clinical trials of R428 have been conducted in patients with metastatic breast cancer, acute myeloid leukemia, and NSCLC [37]. In our study, R428 inhibited erlotinib resistance, colony formation, EMT and migration induced by integrin ß3 in NSCLC cells. It is promising that R428 may be approved for clinical use in the future.
In conclusion, integrin ß3 promotes resistance to EGFR-TKI in NSCLC by upregulating the expression of AXL through the YAP pathway. Patients with advanced NSCLC bearing EGFR mutations and positive for integrin ß3 may benefit from a combination of AXL inhibitors and EGFR-TKI therapy.
Clinical impact of STK11 mutation in advanced-stage non-small cell lung cancer https://www.ejcancer.com/article/S0959-8049(22)00305-7/fulltext
Saken er noen dager gammel, fra 24. juni. Kan være grei å få med seg nå som BerGenBio har laserfokus på blant annet STK11. Kun abstract er gratis tilgjengelig, men til gjengeld er det veldig kort og faktabasert, så kjapt å få lest. OS (overall survival) under overskriften “Results” viser svært mye kortere levetid for dem med STK11 mutasjonen uavhengig av hvilken behandling de fikk, samt at TTF (time to treatment failure) for dem med STK11 var veldig mye kortere. Jeg tror Olin har valgt rett i laserfokus på STK11 og covid.
På dagens liste så har KLP kun 17k igjen, så da får vi anta de er ute , så får vi satse på at resten av fondene holder
Ja, eller øker…
Øker gjør også Covid-smitten tydeligvis - er en smittsom jævel vi har med å gjøre nå.
For BGBIO kan det være en god mulighet dette, mens vi venter på STK11
Vi har nå entret Q3 og signalet (Early 2nd half) fra Olin, betyr at fra nå kan det forventes en snarlig melding om at rekrutteringen i EU S har startet. Slik som Covid smitten ser ut til å utvikles kan rekrutteringen/doseringen være ferdig tidligere enn forventet. Er Covidsatsingen en innertier fra Olin?
I det korte bildet, som selvfølgelig påvirker det lange så tror jeg flere og flere ser at det nå er en bra inngang i BGBIO
- Stigende trend Covid (i denne sammenhengen positivt)
- Oppstart i EU Solid Act er nå, både Trøseid (OUS) og Olin har guidet på dette
- STK11 fremstår mer og mer riktig basert på artikler rundt effekter og påvirkning og ref roadmap på fremdrift vil vi ila tidlig høst få mer info om trials, oppstart og første pasienter inn
- Aksjen har vært tynget av rebalansering hos KLP som nå er ferdigsolgt - det BØR gir noe lettelse riktig vei de neste ukene, selv uten nyheter
- Flere fond har handlet de siste ukene, vi får se om de øker de neste. Det har dog vært mindre posisjoner så langt (MP Pensjon, BNP)
- Hvem sitter bak JP Morgan posten som har økt jevnt og trutt de siste månedene? Nå samlet 2,1 mill aksjer fordelt på 2 konti
Ønsker god sommer folkens 
Face masks reintroduced by Southampton hospital bosses
(Covid: Face masks reintroduced by Southampton hospital bosses - BBC News)
Dere fikk med dere at han som lå med ca 58.000 på kjøp bare ble utålmodig og dundret inn…
Interessant - blir dog overrasket om vi ender over 12, men det er en ivrig 2’er som er tilbake så vi får se 
Sluttkurs 12,15 slettes ikke dårlig.
Vi liker den ivrige 2’er, men vi skulle gjerne hatt flere overivrige 58000’er! Fin børsavslutning. God helg 
In PBMC, the most frequently central genes are associated with COVID-19 severity. Importantly, relative to differential genes, PathExt-identified genes show greater concordance with several benchmark anti-COVID-19 target gene sets. We propose six novel anti-SARS-CoV-2 targets ADCY2, ADSL, OCRL, TIAM1, PBK, and BUB1, and potential drugs targeting these genes, such as Bemcentinib, Phthalocyanine, and Conivaptan.
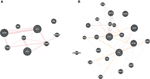
Bemcentinib has been shown to be effective against SC2 (66) and is being tested (trial NCT04890509) for efficacy in hospitalized covid-19 patients. Bemcentinib was designed for targeting AXL, a tyrosine kinase which signals via PI3K (67); however, our analysis identifies it as a lead molecule against ADSL and PBK. We use GeneMANIA software (68) to probe potential relationship between ADSL or PBK with AXL. As shown in Figure 8A, there is a physical interaction between AXL and PBK via PIK3R2. Likewise, interaction can be seen among AXL and ADSL in Figure 8B, suggesting that Bemcentinib’s effect may be mediated by multiple genes within a closely linked gene module. Our computationally generated hypotheses however need experimental validations through knockout or induction studies.
FIGURE 8
Figure 8 Drug-target association. Based on our virtual screening study, we identified, Bemcentinib as a potential inhibitor for PBK and ADSL. Bemcentinib is a well-known drug against AXL, and we saw that this gene (AXL) is connected to our proposed target PBK (A) and ADSL (B) suggesting that the drug effect may be mediated by multiple genes within a closely linked gene module.
Nye artikler med AXL innhold kommer hver helg via Google Scholar. Denne er fra Kina og viser at de er opptatt av hvordan AXL påvirker Covid-19
Bemcentinib er en del av underlaget som reference nr 25.
Fint om noen med mer medisinsk kompetanse kunne trukket essensen ut av denne i et kort summary.
Mipasetamab Uzoptirine (ADCT-601) som er utlisensiert fra BerGenBio til ADC Therapeutics hadde planlagt start av sin fase 1-studie i juni 2022: A Dose-Escalation and Dose-Expansion Study of Mipasetamab Uzoptirine (ADCT-601) in Participants With Solid Tumors - Tabular View - ClinicalTrials.gov
Estimated Study Start Date: June 2022
Så her burde det komme melding om første pasient dosert når som helst. Jeg er spent på å se om BerGenBio også kommer til å sende melding om dette.
Legger ved denne tweeten for dem som vil lese seg opp på utviklingen av ADCT-601: https://twitter.com/MCT_AACR/status/1521497333577535488
Well done @anon95652491 - her er hva som står rundt avtalen med ADC fra prospectet til emisjonen i juli 2020.
Er dette en ny phase 1 eller den samme BGBIO fikk USD 1 mill for ved “dosing of the 5th patient” i 2019?
Er den ny så kan det være sannsynlig at nye USD 1 mill tilfaller BGBIO igjen snart.
Totalt milestone payments PER AXL ADC PRODUCT er på USD 34,25 mill fordelt slik:
- Development and regulatory milestones payments: USD 13,25 mill
- Salesbased milestones payments USD 21 mill
Godt spørsmål. Jeg vet ikke hva kriteriet for “ny” skal være når det gjelder det juridiske i avtalen. Men prøver å legge ved litt ekstra info som kan være relevant for dem som vil vurdere dette:
Tidligere info postet av long41 (bruker nå slettet), viste en kopi av en e-post med spørsmål om at statusen på den “gamle” studien var “terminated”. Her er kopi av e-posten fra ADC Therapeutics fra den gang:
The trial you linked to was terminated in May 2020. We plan to initiate a Phase 2 combination study in the first half of 2022, and that information will be available on clinicaltrials.gov. Mipasetamab uzoptirine is the non-proprietary name for ADCT-601.
I hope that helps to clarify but please let me know if you need anything further.
Så i e-posten sier ADC Therapeutics at den nye studien de skal starte opp i stedet for den gamle som de terminated, at denne nye skal bli en "Phase 2 combination study ", men på lenke til studien nedenfor ser vi at studien er blitt en fase 1-studie, og i “Official Title:”-feltet kaller de det for en “Phase 1b”.
Her er den opprinnelige (gamle) studien som er “terminated”: Safety, Tolerability, Pharmacokinetics, and Antitumor Study of ADCT-601 to Treat Advanced Solid Tumors - Full Text View - ClinicalTrials.gov
Her er lenke til den “nye” studien på nytt, slik at vi har begge lenkene samme sted: A Dose-Escalation and Dose-Expansion Study of Mipasetamab Uzoptirine (ADCT-601) in Participants With Solid Tumors - Tabular View - ClinicalTrials.gov