Photocure ASA: Invitation to presentation of second quarter and first half year 2025 financial results
«APL-1702, a breakthrough therapy for precancerous cervical lesions, is in the final stages before its China launch. Hexvix®, China’s first approved blue-light imaging agent for bladder cancer, marks a new era for photodynamic blue-light cystoscopy.»
« Breakthrough Innovation Poised for Imminent Market Impact
In women’s health, APL-1702, a first-in-class treatment set to debut in China, is on track to become the world’s first non-invasive therapy for high-grade squamous intraepithelial lesion (HSIL) of the cervix, supported by robust clinical evidence and validated in an international Phase III trial. Following the acceptance of its marketing application by China’s National Medical Products Administration (NMPA) in May 2024, the company has made APL-1702 a strategic priority. The regulatory review is progressing smoothly, with the NMPA having initiated a second round of technical evaluation.
APL-1702’s innovation, clinical promise, and societal value have earned strong endorsement from gynecology and pharmaceutical experts. With cervical lesions increasingly affecting younger women in China and current treatments relying on cervical excision, managing precancerous lesions during the critical fertility-preservation window has become a major challenge. Experts believe APL-1702 could address this unmet clinical need, transform the treatment landscape, and support the development of a fertility-friendly society.
To fast-track the commercialization of APL-1702, the company has undertaken multiple pre-launch initiatives. Phase III trial results have been presented at leading academic conferences, and more than 10 expert advisory meetings have been held to support updates to clinical guidelines and consensus. At the China International Import Expo, the product was showcased globally, and the company signed medium- to long-term strategic partnerships with the China Women’s Development Foundation and the Cancer Foundation of China. All of these projects were launched in the first half of 2025. To improve access and affordability, disease-burden and policy research have been conducted. The company is also accelerating the build-out of its commercial team, implementing an omni-channel strategy focused on public hospitals, and optimizing the supply chain to ensure patients can benefit immediately upon approval.
In urologic oncology, Hexvix®, China’s first approved imaging agent for bladder cancer, has ushered in a new era of blue-light cystoscopy. The company is actively supporting its partner R.WOLF in advancing the regulatory review of the SYSTEM BLUE blue-light cystoscopy system in China, with approval targeted by the end of 2025. Once approved, the system, combined with Hexvix®, is expected to improve outcomes for patients nationwide.
To expand market opportunities and strengthen strategic control, the company plans to introduce its single-use blue-light flexible cystoscope to China and accelerate regulatory approval. The aim is to allow patients to fully benefit from blue-light cystoscopy by avoiding unnecessary surgical trauma, preserving bladder function, enhancing quality of life, and reducing total five-year treatment costs through earlier diagnosis.
The company is refining its launch strategy for Hexvix®, initially targeting patients with strong willingness to pay out-of-pocket or coverage through commercial insurance, focusing on urology centers in top-tier and oncology hospitals in major cities. Over time, as clinical guidelines are updated and urology expert endorsements are established, coupled with the launch of single-use blue-light cystoscopes, the company plans to gradually expand penetration into urology centers across key hospitals nationwide, achieving broad access to blue-light cystoscopy.»
«In women’s health, breast cancer, and gynecologic oncology, the results of the international, multicenter Phase III trial of APL-1702 were selected for an oral presentation at the 2025 International Photodynamic Association (IPA) World Congress. The company has also reached agreement with the U.S. FDA on the design of another Phase III trial to support potential U.S. approval of APL-1702 and is seeking overseas partners to prepare for the U.S. IND submission»
«Dr. Kevin Pan, Founder, Chairman and CEO of Asieris Pharmaceuticals, commented, “In the first half of 2025, the company reached multiple milestones, steadily fulfilling our growth expectations. A remarkable achievement is the smooth progression of the regulatory review for APL-1702, our breakthrough therapy for precancerous cervical lesions, now entering the final stages ahead of its China launch and set to benefit patients soon.»
Photocure Partner Asieris publishes Phase III Results from the APRICITY Study for Cevira (APL-1702) in Med journal
Asieris Pharmaceuticals (Stock Code: 688176.SH), announced today that the international multicenter phase III clinical study data of its non-surgical treatment candidate for cervical High-Grade Squamous Intraepithelial Lesion (HSIL) product APL-1702 (Cevira®) have been published online in Med, a flagship medical journal from Cell Press.
Cevira is currently undergoing regulatory review in China.
Read Asieris’ full media release here: https://asieris.com/asieris-publishes-findings-from-the-apricity-study-a-multicenter-phase-iii-global-clinical-trial-of-apl-1702-in-cell-press-journal-med/
About Cevira® (APL-1702) and Asieris
Based on the principles of photodynamic therapy, Cevira® (APL-1702) is undergoing clinical development for use as a photosensitizer in combination with light activation for the non-surgical treatment of high-grade squamous intraepithelial lesions (HSIL) in patients aged 18 years and above, excluding carcinoma in situ. Photocure developed Cevira through Phase I and Phase II clinical trials, and in July 2019, Asieris Meditech Co., Ltd licensed from Photocure the worldwide rights to develop and commercialize this drug-device combination product candidate. In November 2020, Asieris initiated a Phase III clinical trial for APL-1702 (Cevira) which achieved its primary endpoint in September 2023 (Clinical trial number: NCT04484415). The new drug application for Cevira was accepted by the National Medical Products Administration (NMPA) in May 2024, and is currently undergoing regulatory review in China.
Asieris Pharmaceuticals(688176.SH) is a global biopharmaceutical company specializing in the discovery, development, and commercialization of innovative drugs for genitourinary tumors and related diseases.
Questions about clinical development, regulatory application, or commercial strategy for APL-1702 (Cevira) should be directed to Asieris. Investors - Asieris Pharmaceuticals
Norsk:
Photocure-partner Asieris publiserer fase III-resultater fra APRICITY-studien for Cevira (APL-1702) i Med journal
Asieris Pharmaceuticals (aksjekode: 688176.SH) kunngjorde i dag at data fra den internasjonale multisenter kliniske fase III-studien av selskapets ikke-kirurgiske behandlingskandidat for cervikal høygradig plateepitelial lesjon (HSIL) APL-1702 (Cevira®) har blitt publisert på nett i Med, et flaggskipmedisinsk tidsskrift fra Cell Press.
Cevira gjennomgår for tiden regulatorisk vurdering i Kina.
Les hele pressemeldingen til Asieris her: https://asieris.com/asieris-publishes-findings-from-the-apricity-study-a-multicenter-phase-iii-global-clinical-trial-of-apl-1702-in-cell-press-journal-med/
Om Cevira® (APL-1702) og Asieris
Basert på prinsippene for fotodynamisk terapi, er Cevira® (APL-1702) under klinisk utvikling for bruk som fotosensibilisator i kombinasjon med lysaktivering for ikke-kirurgisk behandling av høygradige plateepiteliale intraepiteliale lesjoner (HSIL) hos pasienter i alderen 18 år og eldre, unntatt karsinom in situ. Photocure utviklet Cevira gjennom kliniske studier i fase I og fase II, og i juli 2019 lisensierte Asieris Meditech Co., Ltd fra Photocure de globale rettighetene til å utvikle og kommersialisere denne kombinasjonskandidaten for legemiddel og enhet. I november 2020 startet Asieris en klinisk fase III-studie for APL-1702 (Cevira), som oppnådde sitt primære endepunkt i september 2023 (klinisk studienummer: NCT04484415). Søknaden om det nye legemidlet for Cevira ble godkjent av National Medical Products Administration (NMPA) i mai 2024, og er for tiden under regulatorisk vurdering i Kina.
Asieris Pharmaceuticals (688176.SH) er et globalt biofarmasøytisk selskap som spesialiserer seg på oppdagelse, utvikling og kommersialisering av innovative legemidler for svulster i kjønnsorganer og relaterte sykdommer.
Spørsmål om klinisk utvikling, regulatorisk søknad eller kommersiell strategi for APL-1702 (Cevira) bør rettes til Asieris. Investorer - Asieris Pharmaceuticals
PHOTOCURE GOES AI
Exploration of Novel AI-enabled BlueLight Enhanced Cystoscopy (ENAiBLE)
Study Overview
Brief Summary
Blue light cystoscopy (BLC) is a diagnostic procedure in bladder cancer where the inside of the bladder is observed with a camera to detect bladder lesions. Unlike regular white lightcystoscopy, blue light cystoscopy takes use of a drug that induces fluorescence under bluelight preferentially in neoplastic and malignant cells that helps visualize bladder lesions during the cystoscopic procedure. Blue lightcystoscopy has shown to improve detection of bladder cancer. The purpose of the study is to collect images, video and associated pathology results from blue light cystoscopy procedures to explore potential of and develop an AI decision support tool. The decision support tool is hypothesized to further improve the diagnostic accuracy of the blue light procedure.
https://clinicaltrials.gov/study/NCT07144319?term=blue%20light%20cystoscopy&rank=3
Official Title
Exploration of Novel AI-enabled Blue LightEnhanced Cystoscopy
Conditions
Data Collection for AI Development
Other Study ID Numbers
- PCAIX01/25
Study Start (Estimated)
2025-09
Primary Completion (Estimated)
2026-12
Blue light cystoscopy (BLC) is a diagnostic procedure in bladder cancer where the inside of the bladder is observed with a camera to detect bladder lesions. Unlike regular white light cystoscopy, blue light cystoscopy takes use of a drug that induces fluorescence under blue light preferentially in neoplastic and malignant cells that helps visualize bladder lesions during the cystoscopic procedure. Blue light cystoscopy has shown to improve detection of bladder cancer. The purpose of the study is to collect images, video and associated pathology results from blue light cystoscopy procedures to explore potential of and develop an AI decision support tool. The decision support tool is hypothesized to further improve the diagnostic accuracy of the blue light procedure.
Skal kjøres på 500 pasienter.
Dette innlegget ble rapportert og er midlertidig skjult.
Jeg er trolig mest blasert av AI-hypen av alle her inne, men her er det dels tvingende nødvendig at de tar eierskap for hva AI kan (eller mest sannsynlig: ikke kan) gjøre for denne typen billeddiagnostikk.
Dette er jo for å møte konkurransen fra hvitt lys kombinert med AI og som leverer bedre resultater enn hvitt lys uten AI. Om hvitt lys med AI nærmer seg BLC uten AI kan det bli vanskelig å selge inn BLC som jo har en tyngre prosedyre enn hvitt lys. Dette ble diskutert på tråden for ett til to år siden, så det er ikke noe nytt.
AI er jo faktisk veldig bra på bildediagnostikk, noe som er etablert standard innen flere områder. For eksempel har man på enkelte sykehus i Norden erstattet den ene av de to radiologene som gransker bildene med AI siden kliniske forsøk viser at resultatene blir bedre (og man frigjør ressurser/øker kapasiteten).
Det er også gjort forsøk på AI med hvitt lys som viser gode resultater.
Nøkkelen er å ha ett kontrollert datasett av tilstrekkelig størrelse å øve AIen på. Det burde PHO ha, eller skaffe seg fra sine kunder.
Link nederst:
The presentation will be held in English and questions can be submitted throughout the event. The streaming event is available through: https://channel.royalcast.com/landingpage/hegnarmedia/20251029_2/
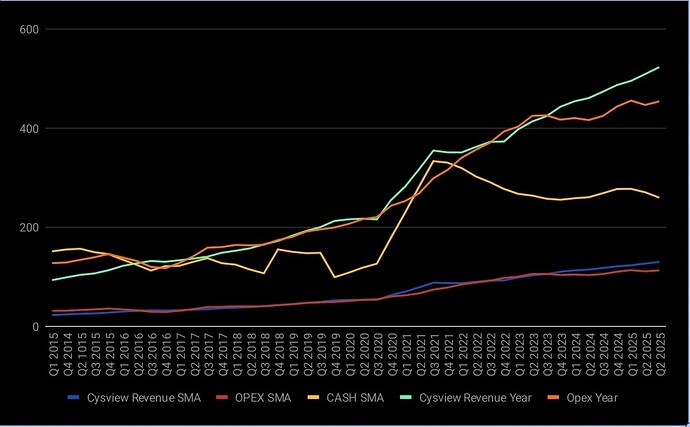
Core business på Cysview vokser, men den vokser veldig sakte.
Her er 4 kvartals glidende snitt, og sum 4 siste kvartaler. Cysview revenue trenger å stige fortere, og opex trenger å holde seg mer eller mindre flatt:
Dette var en svak G+ rapport for Q3, ikke i nærheten av noen M.
Håper de snart bruker nye 50 millioner på tilbakekjøp, ser ingen grunn til at de trenger å sitte på hundrevis av millioner.
Norne
Photocure delivered yet another solid quarter, with 3Q figures broadly exceeding our estimates. The company continued to post double-digit YoY growth on the top line while maintaining well-contained opex. Reported EPS came in above our expectations, and the cash position remains solid. We believe this robust financial performance further underpins our investment case on the stock going forward.