Begynner å bli noen publikasjoner:
April 2017:
 SpringerLink
SpringerLink

In newly diagnosed metastatic hormone-naive prostate cancer (mPC), telomerase-based immunotherapy with the novel hTERT peptide vaccine UV1 can induce immune responses with potential clinical benefit. This phase I dose escalation study of UV1…
Treatment with UV1 and GM-CSF gave few adverse events and induced specific immune responses in a large proportion of patients unselected for HLA type. The intermediate dose of 0.3 mg UV1 resulted in the highest proportion of, and most rapid UV1-specific immune responses with an acceptable safety profile. These results warrant further clinical studies in mPC.
November 2020:
 Frontiers
Frontiers
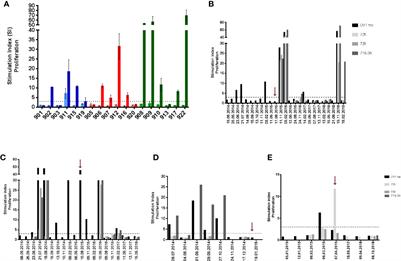
Human telomerase reverse transcriptase (hTERT) is a target antigen for cancer immunotherapy in patients with non-small cell lung cancer (NSCLC). We have tested a novel hTERT vaccine, UV1, designed to give high population coverage. UV1 is composed of…
Treatment with UV1 was well tolerated with no serious adverse events observed. Seventeen patients were evaluable for tumor response; 15 patients had stable disease as best response. The median progression free survival (PFS) was 10.7 months, and the median overall survival (OS) was 28.2 months. The OS at 4 years was 39% (7/18). Five patients are alive (median survival 5.6 years), and none of these are known to have received checkpoint therapy after vaccination. UV1 induced specific T-cell responses in the majority (67%) of patients. Immune responses were dynamic and long lasting. Both immune response (IR) and OS were dose related. More patients in the highest UV1 dosage group (700 μg) developed IRs compared to the other groups, and the IRs were stronger and occurred earlier. Patients in this group had a 4-year OS of 83%. The safety and clinical outcome data favor 700 μg as the preferred UV1 dose in this patient population. These results provide a rationale for further clinical studies in NSCLC with UV1 vaccination in combination with immune checkpoint blockade.
Mai 2021:
 Frontiers
Frontiers
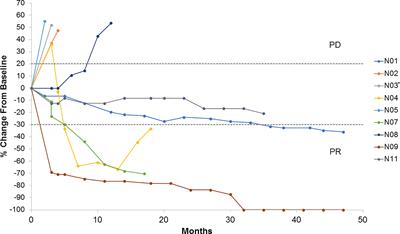
BackgroundIpilimumab improves survival for patients with metastatic malignant melanoma. Combining a therapeutic cancer vaccine with ipilimumab may increase efficacy by providing enhanced anti-tumor immune responses. UV1 consists of three synthetic…
Twelve patients were recruited. Adverse events were mainly diarrhea, injection site reaction, pruritus, rash, nausea and fatigue. Ten patients showed a Th1 immune response to UV1 peptides, occurring early and after few vaccinations. Three patients obtained a partial response and one patient a complete response. Overall survival was 50% at 5 years. Treatment was well tolerated. The rapid expansion of UV1-specific Th1 cells in the majority of patients indicates synergy between UV1 vaccine and CTLA-4 blockade. This may have translated into clinical benefit, encouraging the combination of UV1 vaccination with standard of care treatment regimes containing ipilimumab/CTLA-4 blocking antibodies.
Mai 2021:
 BioMed Central
BioMed Central

Background Malignant pleural mesothelioma (MPM) is a rare and aggressive tumour. For patients with inoperable disease, few treatment options are available after first line chemotherapy. The combination of ipilimumab and nivolumab has recently shown…
Across the three phase I/II-studies conducted with UV1, vaccine-specific immune responses were observed in 78% (range 67–91%) of patients across the different cancer types (and HLA allele types), supporting the universality of the vaccine. Survival time for patients who responded immunologically was longer than for patients who did not respond immunologically to the vaccine, and survival time correlated with the breadth of the vaccine-specific immune response […] In summary, UV1 is safe alone and in combination with checkpoint inhibitors and trials in other cancers show that UV1 induces vaccine-specific immune response associated with survival.
Juli 2021:
 Frontiers
Frontiers
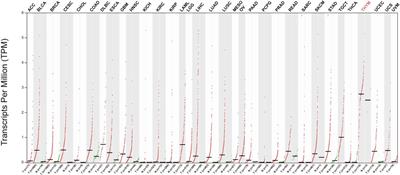
Telomerase-based therapeutic cancer vaccines (TCVs) have been under clinical investigation for the past two decades. Despite past failures, TCVs have gained renewed enthusiasm for their potential to improve the efficacy of checkpoint inhibition…
Telomerase as a TCV target has apparent advantages due to its universal presence and essential function in almost all cancer types, providing spatiotemporal relevance to the induced immune response and limiting possible escape mechanisms for the tumor.
[…]
Immunologically rational combinations, such as anti-CTLA-4 and anti-PD-1/L1, are likely necessary to bring out the true clinical potential of hTERT-targeting TCVs. There are already several phase II randomized controlled trials evaluating hTERT targeting TCVs in combination with CPIs with anticipated read-outs.
Mai 2022:
 Journal for ImmunoTherapy of Cancer – 1 May 22
Journal for ImmunoTherapy of Cancer – 1 May 22
Background Therapeutic cancer vaccines represent a promising approach to improve clinical outcomes with immune checkpoint inhibition. UV1 is a second generation telomerase-targeting therapeutic cancer vaccine being investigated across multiple…
In total, 78.4% of treated patients mounted a measurable vaccine-induced T cell response in blood. The immune responses in the malignant melanoma trial, where UV1 was combined with ipilimumab, occurred more rapidly and frequently than in the lung and prostate cancer trials. In several patients, immune responses peaked years after their last vaccination. An in-depth characterization of the immune responses revealed polyfunctional CD4+ T cells producing interferon-γ and tumor necrosis factor-α on interaction with their antigen. Long-term immunomonitoring of patients showed highly dynamic and persistent telomerase peptide-specific immune responses lasting up to 7.5 years after the initial vaccination, suggesting a plausible functional role of these T cells in long-term survivors. The superior immune response kinetics observed in the melanoma study substantiate the rationale for future combinatorial treatment strategies with UV1 vaccination and checkpoint inhibition for rapid and frequent induction of anti-telomerase immune responses in patients with cancer.
September 2022:
 BioMed Central
BioMed Central

Background This clinical trial evaluated a novel telomerase-targeting therapeutic cancer vaccine, UV1, in combination with ipilimumab, in patients with metastatic melanoma. Translational research was conducted on patient-derived blood and tissue…
Vaccine-specific immune-responses were detected in 91% of evaluable patients. Clinical responses were observed in four patients. The mPFS was 6.7 months, and the mOS was 66.3 months. There was no association between baseline tumor mutational burden, neoantigen load, IFN-γ gene signature, tumor-infiltrating lymphocytes, and response to therapy. Tumor telomerase expression was confirmed in all available biopsies. Vaccine-enriched TCR clones were detected in blood and biopsy, and an increase in the tumor IFN-γ gene signature was detected in clinically responding patients. Clinical responses were observed irrespective of established predictive biomarkers for checkpoint inhibitor efficacy, indicating an added benefit of the vaccine-induced T cells. The clinical and immunological read-out warrants further investigation of UV1 in combination with checkpoint inhibitors.
Januar 2023:
 LWW
LWW

ve durable benefit from CPIs, and improvements are urgently needed. The clinical efficacy of CPIs depends on highly variable preexisting spontaneous T-cell immune responses. Cancer vaccines represent an independent treatment modality uniquely capable…
Key points
- The clinical efficacy of checkpoint inhibitors depends on preexisting, spontaneous immune responses against patients’ tumors.
- Anti-hTERT immune responses appear beneficial for melanoma patients, and telomerase represents an attractive target for vaccination.
- Cancer vaccines represent an independent mode of action capable of increasing cancer patient repertoire of tumor specific T cells.
-
Survival data are expected imminently from INITIUM, a randomized phase II clinical trial ( n = 156) in metastatic melanoma comparing the effects of treatment with the standard-of-care checkpoint inhibitors, ipilimumab and nivolumab, with and without the telomerase vaccine, UV1.
In the near future, randomized data from clinical trials involving therapeutic cancer vaccines and checkpoint inhibitors will be available. Positive readout may spark broad development and allow cancer vaccines to find their place in the clinic as an important component in multiple future CPI combinations.

















